Biofilms are communities of microbes that attach to surfaces, creating a protective structure that can be resistant to detergents, drying, and other stresses.1
Biofilms can form in all endoscope channels, but narrow channels are particularly susceptible.2,3 These channels are not able to be brushed during manual cleaning, allowing biofilm to proliferate in them despite reprocessing.
Multiple studies show that clinically used endoscopes can harbour biofilm
Persistently contaminated endoscopes can act as reservoirs and harbour biofilm, even after reprocessing.4,5
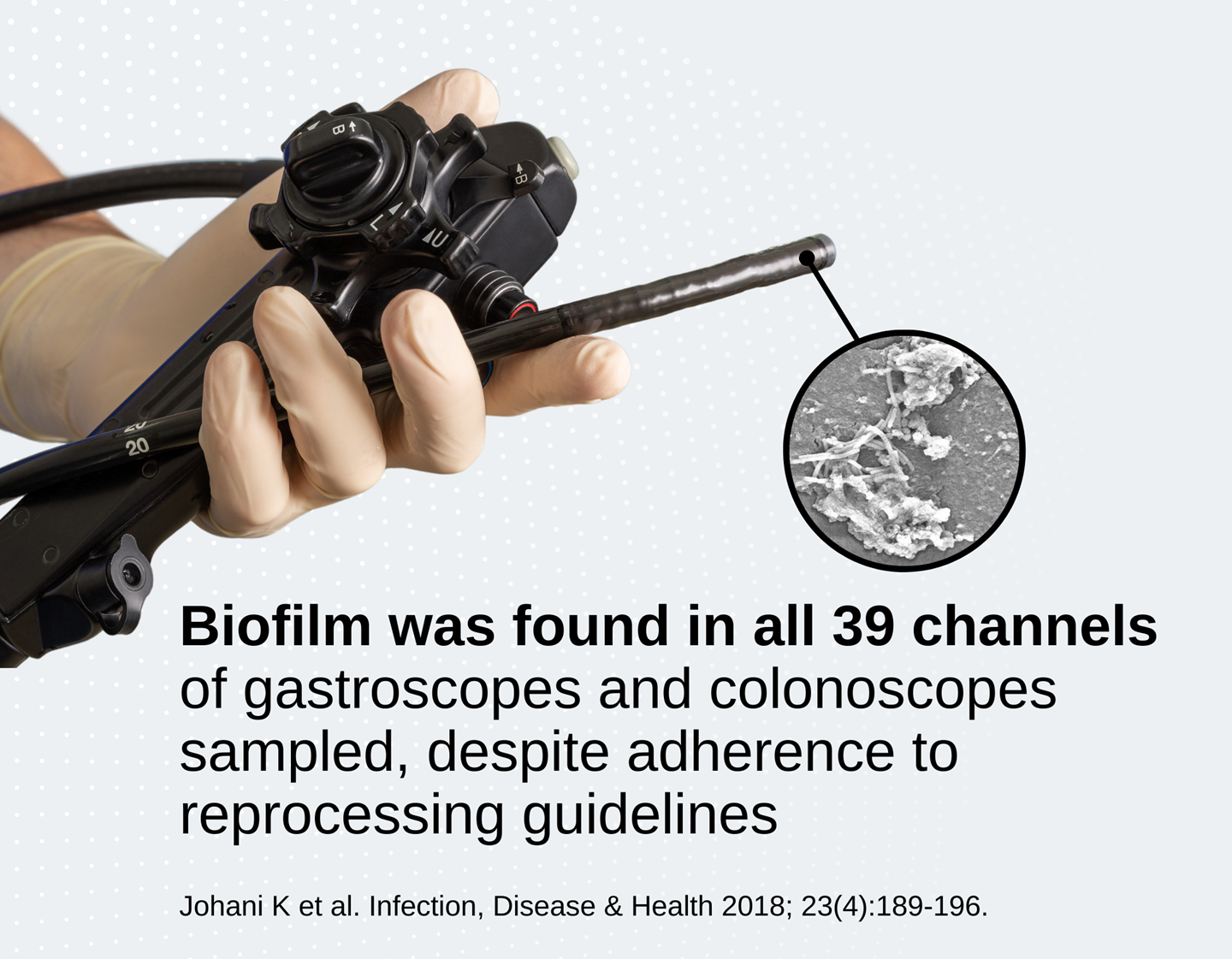
In a clinical study, the majority of gastroscopes had biofilm in multiple channels after 60 days of patient use/reprocessing cycles5
Microbes present in the environment, including those in contaminated endoscopes in clinical use, predominantly exist in biofilms.1 Tests to assess the efficacy of endoscope cleaning and reprocessing must therefore include models that simulate biofilms.
Biofilm is resistant to high-level disinfection
It is essential that biofilm is removed during the manual cleaning stage, as evidence suggests it may be able to survive high-level disinfection.6 This resistance can develop further over time as residual dead cells (biomass) accumulates.7
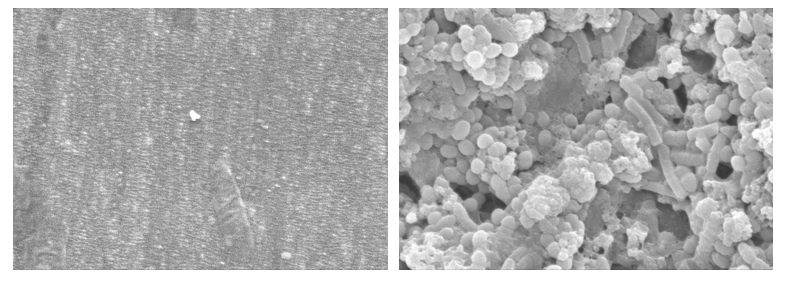 Comparison of 1.4mm PTFE tube (to simulate air/water and auxiliary channels) without biofilm (left) and with biofilm (right). 8000X magnification.
Comparison of 1.4mm PTFE tube (to simulate air/water and auxiliary channels) without biofilm (left) and with biofilm (right). 8000X magnification.
Resistant strains of biofilm are also emerging. Carbapenem-resistant enterobacterales (CRE) show increased tolerance to peracetic acid (PAA) when grown as a biofilm.8 K. pneumoniae isolated from an outbreak involving a duodenoscope showed weak resistance to PAA in its planktonic (free-living) form. When grown as a biofilm, the bacteria showed resistance to PAA even at a concentration used for high-level disinfection.
Biofilm can cause outbreaks over time
Multiple studies have demonstrated that a single strain of bacteria can be transmitted to patients via the same endoscope, over a period of months:
- 27 patients were colonized with multidrug-resistant Klebsiella pneumonia, with 10 developing an active infection. The outbreak was traced to two endoscopes persistently contaminated with K. pneumoniae over 8 months.9
- One endoscope infected multiple patients with the same strain of Pseudonomas aeruginosa. Biofilm was present in undamaged channels and could not be removed despite repeated HLD10
These reports show that endoscopes can act as reservoirs for pathogens, despite repeated cleaning and reprocessing.
Given this evidence, it is likely that the formation of protective biofilms allows pathogens to persist in endoscopes over time.

Endoscope reprocessing can cause biofilm to become more resistant to removal
Multiple cycles of reprocessing and drying can cause endoscopes to develop a tougher form of biofilm that can be more difficult to remove.11,12
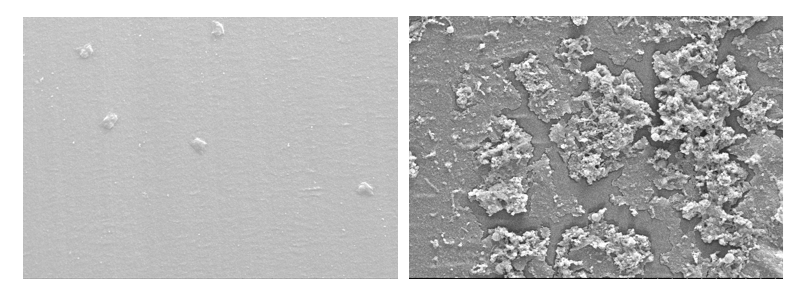 Comparison of 3.7mm PTFE tube (to simulate suction biopsy channel) without biofilm (left) and with biofilm (right). 1000X magnification.
Comparison of 3.7mm PTFE tube (to simulate suction biopsy channel) without biofilm (left) and with biofilm (right). 1000X magnification.
Biofilm found in endoscopes in clinical use is often fixed to the surface from repeated cycles of HLD and drying, meaning mechanical action is critical for its removal.11 It may be impossible to remove this biofilm even after multiple rounds of standard cleaning and disinfection protocols, requiring the endoscope channel to be replaced.13
Cyclic-buildup biofilm accurately models clinical conditions
The cyclic-buildup biofilm model, developed by Alfa et al.10, represents the multiple cycles of HLD and drying that can cause biofilm to become fixed. This model is the closest available representation of biofilm isolated from endoscopes in clinical use.
Traditional models are usually single-organism biofilms that are continuously hydrated, and do not model the growth of biofilm in channels of different diameters.14
|
Clinical Biofilm (isolated from contaminated endoscopes) |
Cyclic-buildup |
Traditional Biofilm 14 |
|
|
Includes potential GI pathogens |
✓ |
✓ |
✓ |
|
Grows in polytetrafluoroethylene (PTFE) channels |
✓ |
✓ |
✓ |
|
Consists of multiple organisms |
✓ |
✓ |
- |
|
Grows over >5 days |
✓ |
✓ |
- |
|
Subjected to multiple cycles of drying/wetting |
✓ |
✓ |
- |
|
Crosslinking of amino acids induced by fixative chemicals |
|
✓ |
- |
|
Cycles of reinoculation |
✓ |
✓ |
- |
|
Grows in both small and large channels |
✓ |
|
- 6mm diameter tubing only |
*Where glutaraldehyde (GTA) and ortho-phthalaldehyde (OPA) are used
- Roberts CG. The role of biofilms in reprocessing medical devices. Am J Infect Control. 2013 May;41(5 Suppl):S77-80. doi: 10.1016/j.ajic.2012.12.008.
- Kovaleva J Infectious complications in gastrointestinal endoscopy and their prevention. Best Pract Res Clin Gastroenterol. 2016 Oct;30(5):689-704.
- Ren-Pei et al. Correlation between the growth of bacterial biofilm in flexible endoscopes and endoscope reprocessing methods. Am J Infect Control. 2014 Nov;42(11):1203-6.
- Johani K et al. Determination of bacterial species present in biofilm contaminating the channels of clinical endoscopes. 2018; 23(4):189-196.
- Primo MGB, et al. Biofilm accumulation in new flexible gastroscope channels in clinical use. Infect Control Hosp Epidemiol. 2021; 15:1-7.
- Akinbobola AB, et al. Tolerance of Pseudomonas aeruginosa in in-vitro biofilms to high-level peracetic acid disinfection. J Hosp Infect. 2017;97(2):162-168. doi:
- Akinbobola AB, et al. ‘Secondary biofilms’ could cause failure of peracetic acid high-level disinfection of endoscopes. J Hosp Infect 2021; 107:65-75.
- Brunke MS. Tolerance of biofilm of a carbapenem-resistant Klebsiella pneumoniae involved in a duodenoscopy-associated outbreak to the disinfectant used in reprocessing. Antimicrob Resist Infect Control. 2022; 11(1):81.
- Rauwers et al. Independent root cause analysis of contributing factors, including dismantling of 2 duodenoscopes, to an outbreak of multidrug-resistant Klebsiella pneumoniae. Gastrointest Endosc. 2019; 90(5):793-804.
- Kovaleva, J. et al. Is bacteriologic surveillance in endoscope reprocessing stringent enough? Endoscopy 2009; 41:913–916.
- Ribeiro MM, Graziano KU, Olson N, França R, Alfa MJ. The polytetrafluoroethylene (PTFE) channel model of cyclic-buildup biofilm and traditional biofilm: The impact of friction, and detergent on cleaning and subsequent high-level disinfection. Infect Control Hosp Epidemiol. 2020; 41(2):172-180.
- Qiu L, Zhou Z, Liu Q, Ni Y, Zhao F, Cheng H. Investigating the failure of repeated standard cleaning and disinfection of a Pseudomonas aeruginosa-infected pancreatic and biliary endoscope. Am J Infect Control 2015;43:e43–e46
- Alfa MJ & Howie R. Modeling microbial survival in buildup biofilm for complex medical devices. BMC Infect Dis 2009; 9:56.
- International Standards Organization. ISO 15883-5:2021 Washer-disinfectors — Part 5: Performance requirements and test method criteria for demonstrating cleaning efficacy. (2021).

