Healthcare-associated infections (HAIs) are infections associated with the treatment of patients in healthcare settings, as direct or indirect result of healthcare.1,2 This means a patient acquired a new infection from their healthcare stay or treatment.
The Most Frequent Medical Adverse Event
HAIs are the most frequently occurring medical adverse event worldwide, with a prevalence of around 7-10%.3,4
There can be serious physical and emotional impacts to patients that acquire an HAI.2 Facilities and practitioners may also suffer financial as well as reputational damage as a result of HAIs associated with their facility.
HAIs Add Considerable Cost to Delivering Healthcare
The Australian Commission on Safety and Quality in Health Care (ACSQHC) estimates 11,142 fewer HAIs would result in a possible value capture of $459,984,691.5
HAIs are Preventable
The incidence of HAIs in Australia is estimated to be 165,000 per year.6
A systematic review and meta-analysis found 35-55% of HAIs are preventable with existing multifaceted infection prevention and control interventions, regardless of a country’s income level.7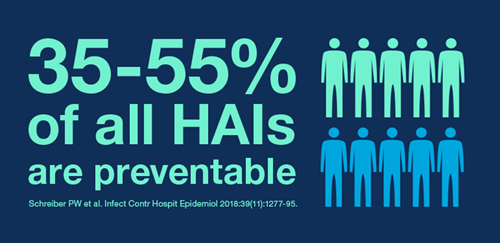
The Role of Medical Devices in HAIs
Sterilisation and disinfection of medical devices play an important role in the prevention of HAIs. Multiple studies document failures in medical device reprocessing, some of which have led to outbreaks and patient exposure.8-10
Population Level Study: Infection Risk after Ultrasound
In the first of its kind, a study commissioned by UK National Health Authorities (Scotland) revealed an "unacceptable risk" of patient infection following ultrasound. The study revealed an increased infection risk in the 30 days following endocavitary ultrasound. Commissioned by a national health authority, the study revealed an increased infection risk in the 30 days following endocavitary ultrasound.11
90.5% of facilities were not performing high level disinfection (HLD) of these probes at the time of the study.
Public Health Risk Reality
Of the 982,911 patients followed, 330,500 were gynaecological patients.
60,698 of these patients had undergone transvaginal ultrasound.

The national health authority now recommends HLD for endocavitary ultrasound probes.
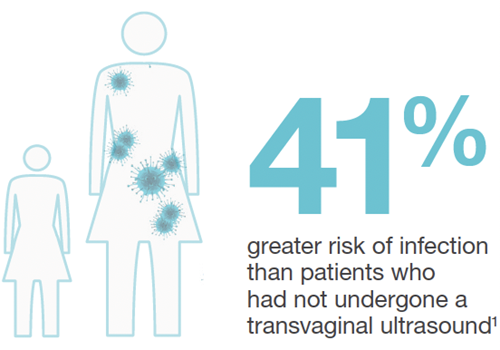
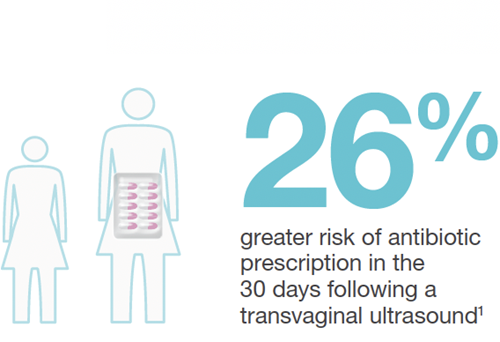
The increased rates of infection risk following transvaginal ultrasound were startling:11
Prevent HAIs from Medical Devices
The Spaulding Classification identifies the level of disinfection or sterilisation required for medical devices based on the expected patient tissue the device will contact.
The Spaulding Classification forms the basis of international ultrasound probe reprocessing guidelines.
Internationally, Federal authorities and professional societies provide guidance on the proper reprocessing of ultrasound probes, to help keep patients safe from HAIs.
These guidelines are based on The Spaulding Classification.
Learn when to HLD ultrasound probes
Learn about the clinical risks from improper ultrasound probe reprocessing
- World Health Organization (WHO) 2020. Infection prevention and control. The burden of health care-associated infection worldwide. Webpage accessed October 2020.
- Australian Commission on Safety and Quality in Health Care (ACSQHC) 2019. Healthcare-Associated Infection Program. Webpage accessed October 2020.
- Currie K, et al. Am J Infect Control. 2018;46(8):936-42.
- World Health Organization (WHO). Healthcare Associate Infections Fact Sheet. Accessed online October 2020.
- ACSQHC 2019. The State of Patient Safety and Quality in Australian Hospitals 2019.
- Mitchell B.G. et al. Infection, Disease and Health. 2017; 22, 117-128
- Schreiber PW, et al. Infect Control Hosp Epidemiol. 2018;39(11):1277-95
- WHO 2016. Decontamination and Reprocessing of Medical Devices for Health-care Facilities.
- CDC 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities.
- Rutala WA, Weber DJ. Infect Dis Clin North Am. 2016 Sep;30(3):609-37.
- Scott D et al. Ultrasound 2018;26(3): 168-177.

