Studies show improper ultrasound probe disinfection practices leave probes contaminated with microorganisms.
- More than 45% of ultrasound probes used across 5 EDs and 5 ICUs had bacterial contamination and over 50% had blood contamination.1
- More than 90% of transvaginal ultrasound probes were contaminated after cleaning with a paper towel and more than 50% tested positive for MRSA or other potentially pathogenic bacteria.2
- More than 80% of ultrasound probe handles remain contaminated when not disinfected.3,4
- A meta analysis found a prevalence of 12.9% for frequently occurring bacteria and 1% for viruses on transvaginal & transrectal probes after low level disinfection (LLD) wipes and sprays.5
- More than 20% of probe heads remained contaminated with bacteria after LLD with wipes.3

The Risks from Improper Ultrasound Probe Reprocessing
Patient safety as well the financial and reputational health of a facility are at risk when best practice reprocessing is not implemented.
Patient Death
A patient died from hepatitis B thought to have been transmitted by an improperly disinfected endocavitary ultrasound probe.6
The UK Medicine and Healthcare Products Regulatory Agency (MHRA) released an alert for healthcare facilities to review all ultrasound disinfection practices in UK.

Epidemiological Study: Infection Risk Found after Ultrasound
Commissioned by a national health authority, the study revealed an increased infection risk in the 30 days following endocavitary ultrasound.7
90.5% of facilities were not performing high level disinfection (HLD) of these probes at the time of the study.
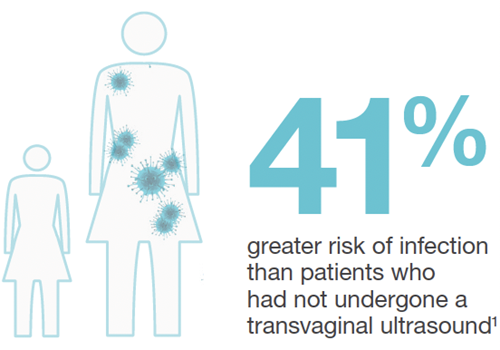
Of the 982,911 patients followed, 330,500 were gynaecological patients. 60,698 of these patients had undergone transvaginal ultrasound. The increased rates of infection risk following transvaginal ultrasound were startling.7
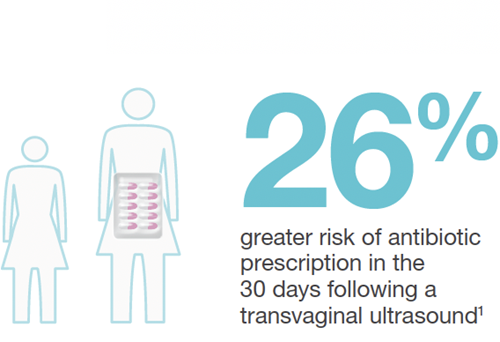
The national health authority now recommends HLD for endocavitary ultrasound probes.
Ultrasound Probe Disinfection
Ultrasound probes need to be properly disinfected before use to mitigate infection transmission risks.
The Spaulding Classification tells us how to disinfect ultrasound probes based on how they will be used and forms the basis of international ultrasound probe reprocessing guidelines. Learn more about The Spaulding Classification and ultrasound probe reprocessing guidelines.
Probe Sheaths do not Replace HLD
Condoms break up to 13% of the time and commercial covers fail up to 5% of the time.8-11 Recent literature reaffirms probe sheaths do not replace the need for HLD.11,12
HLD Outperforms LLD
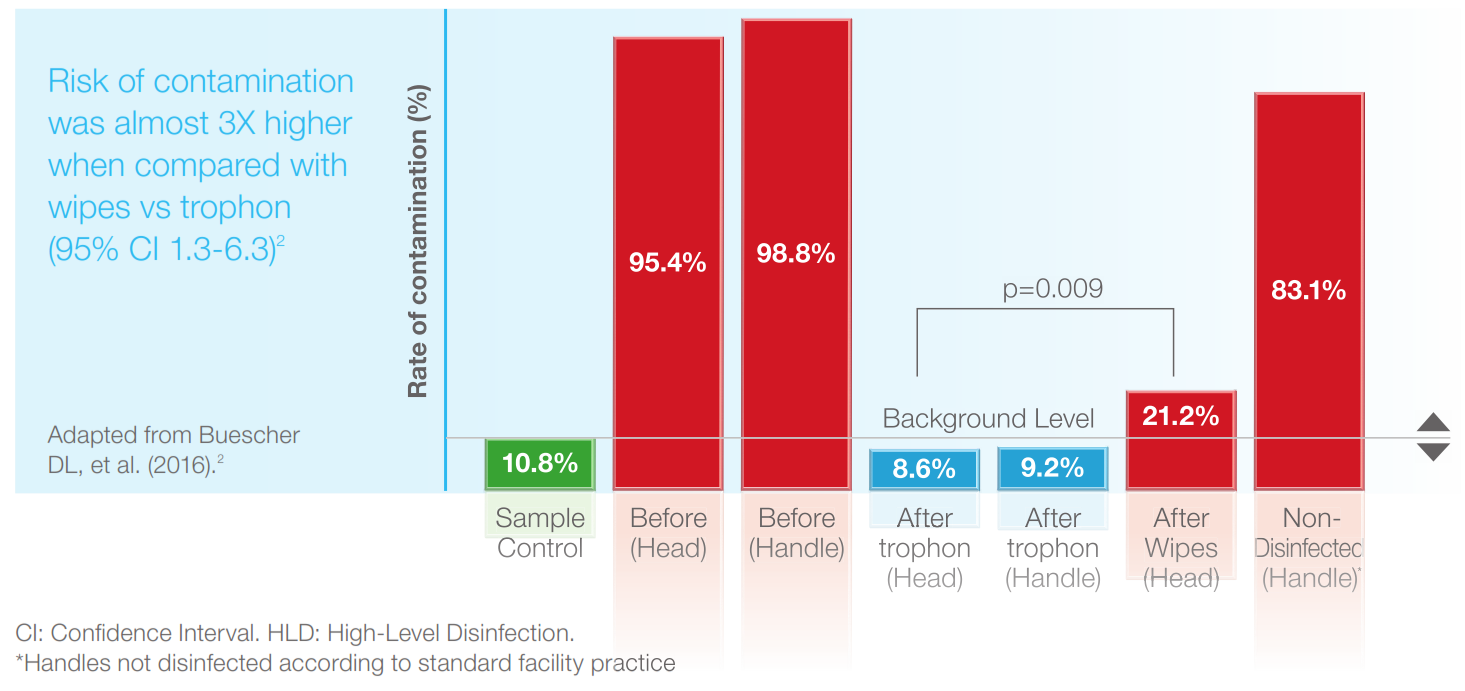
A study sampled transvaginal ultrasound probes after LLD with wipes. >20% of probe heads remained contaminated with bacteria, an organism that should be eliminated by LLD (Figure below).3
Automated HLD with the trophon® device successfully eliminated bacteria in the same study from both the probe heads and handles.3
Learn more about the trophon®2 device
The trophon® family of devices includes the trophon®2 and trophon® EPR which share the same core technology of 'sonically activated' hydrogen peroxide.
References:
- Keys, M., et al. Crit Care Resusc. 2015:17(1): 43-46.
- Oide, S., et al. (2019). J Med Ultrason 46(4): 475-479.
- Buescher DL, et al. Ultrasound Obstet Gynecol 2016;47(5): 646-651.
- Ngu, A., et al. (2015). Infect Control Hosp Epidemiol 36(5): 1-4.
- Leroy S, et al. J Hosp Infect 2013;83(2): 99-106.
- MHRA UK 2012. Medical Device Alert: Reusable transoesophageal echocardiography, transvaginal and transrectal ultrasound probes (transducers) (MDA/2012/037).
- Scott D et al. Risk of infection following semi-invasive ultrasound procedures in Scotland, 2010 to 2016: A retrospective cohort study using linked national datasets. Ultrasound 2018;26(3): 168-177.
- Amis S, et al. J Clin Ultrasound. 2000;28(6):295-8.
- Milki A, et al. Fertil Steril. 1998;69(3):409-11.
- Masood J, et al. Int Urol Nephrol. 2007;39(4):1121-4.
- Basseal JM, et al. Infect Dis Health. 2020;25(2):77-81
- Westerway SC, et al. Ultraschall Med. 2020. doi: 10.1055/a-1168-6602. Online ahead of print.

