Earle H. Spaulding recognised a disinfection framework was required since all reusable medical devices cannot be sterilised.1
The Spaulding Classification met a key unmet need that today still forms the basis of international medical device disinfection guidelines.
The Spaulding Classification
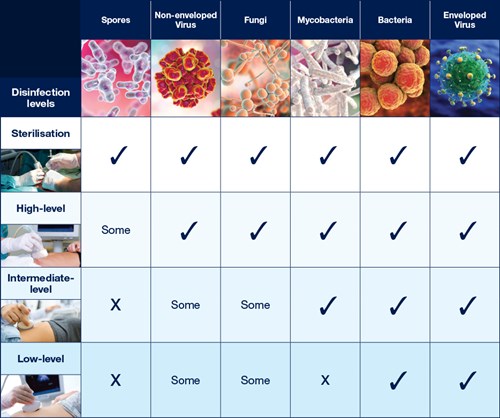
The Spaulding Classification stratifies the risk of infection transmission based on the patient tissue the device will contact during use. The device classification determines the level of disinfection/sterilisation required (Table 1).
Table 1: Overview of The Spaulding Classification.1-6
|
Spaulding Classification |
Medical Device Contacts |
Risk of Infection Transmission |
Disinfection Level |
|
Critical |
Sterile tissue or the bloodstream |
High |
Sterilisation* |
|
Semi-critical |
Mucous membranes or non-intact skin |
Medium |
High Level Disinfection (HLD) |
|
Non-critical |
Intact skin only |
Low |
Intermediate level (ILD) or Low level disinfection (LLD) |
* Critical ultrasound probes can be high level disinfected and used with a sterile sheath if sterilisation is not possible.5,6
Sterilisation
Sterilisation destroys all microorganisms.4
Critical devices must be sterile when used.1
High Level Disinfection (HLD)
HLD destroys all microorganisms with the exception of high numbers of bacterial spores.4 A high level disinfectant is therefore bactericidal, virucidal (both lipid and non-lipid viruses), fungicidal and mycobactericidal.
Semi-critical ultrasound probes must undergo HLD and be used with a sheath.4-5
Critical ultrasound probes that cannot be sterilised can also undergo HLD.4,5 They must also be used with a sterile sheath.4,5
Low & Intermediate Level Disinfection
An intermediate level or low level instrument grade disinfectant may be used for disinfection of non-critical instruments.4
Low level instrument grade disinfectants kill vegetative bacteria, some fungi and some viruses.4
Intermediate level instrument grade disinfectants kill vegetative bacteria, mycobacteria, viruses and most fungi but do not kill bacterial spores.4
Understanding Disinfection
It is important to recognise there are differences between disinfection levels and sterilisation. Correctly applying The Spaulding Classification to medical devices is a key part of keeping patients safe from healthcare-associated infections (HAIs).
References
- Spaulding EH (1968). Chemical disinfection of medical and surgical materials. Disinfection, sterilization, and preservation. Lawrence C, Block SS. Philadelphia (PA), Lea & Febiger: 517-531.
- Australian Commission on Safety and Quality in Health Care (ACSQHC). National Safety and Quality Health Service Standards (NSQHSS). 2 ed. Sydney, Australia: ACSQHC; 2017.
- National Health and Medical Research Council (NHMRC). Australian Guidelines for the Prevention and Control of Infection in Healthcare. Canberra, Australia: NHMRC; 2019.
- AS 5369:2023 Reprocessing of reusable medical devices and other devices in health and non-health related facilities
- Australasian College for Infection Prevention and Control (ACIPC), Australasian Society for Ultrasound in Medicine (ASUM). Guidelines for Reprocessing Ultrasound Transducers. Australasian Journal of Ultrasound in Medicine. 2017;20(1):30-40.
- College of Intensive Care Medicine of Australia and New Zealand (CICM). Prevention of pathogen transmission during ultrasound use in the Intensive Care Unit: Recommendations from the College of Intensive Care Medicine Ultrasound Special Interest Group (USIG). Date accessed: 14th of May 2020.

